Rank the following nuclei in ascending order according to the binding energy per nucleon smallest first. ANucleus D is the most stable and nucleus A is the least stable.
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html
Nucleus III is the most stable and nuclei I and V are the least stable E.

. Nuclei with even numbers of protons neutrons or both are more likely to be stable see Table 1. An isotope of hydrogen viz. Heavy nuclei with an even number of protons and an even number of neutrons are due to Pauli exclusion principle very stable thanks to the occurrence of paired spin.
All stable isotopes stable by observation not theory are the ground states of nuclei with the exception of tantalum-180m which is a nuclear isomer or excited state. C The curve has certain peaks indicating that certain nuclei like C He 12 6 4 2 and O 16 8 are much more stable than the nuclei in their vicinity. The correct order of True False in above statements is.
Alpha particles α Beta particles β Gamma particles γ. What nuclei would be the least stable. The correct answer would be option D.
Nuclides containing odd numbers of both protons and neutrons are the least stable and this means more radioactive. 1 proton 3 neutrons. The isotopes with intermediate mass numbers 40 to 100 are most stable.
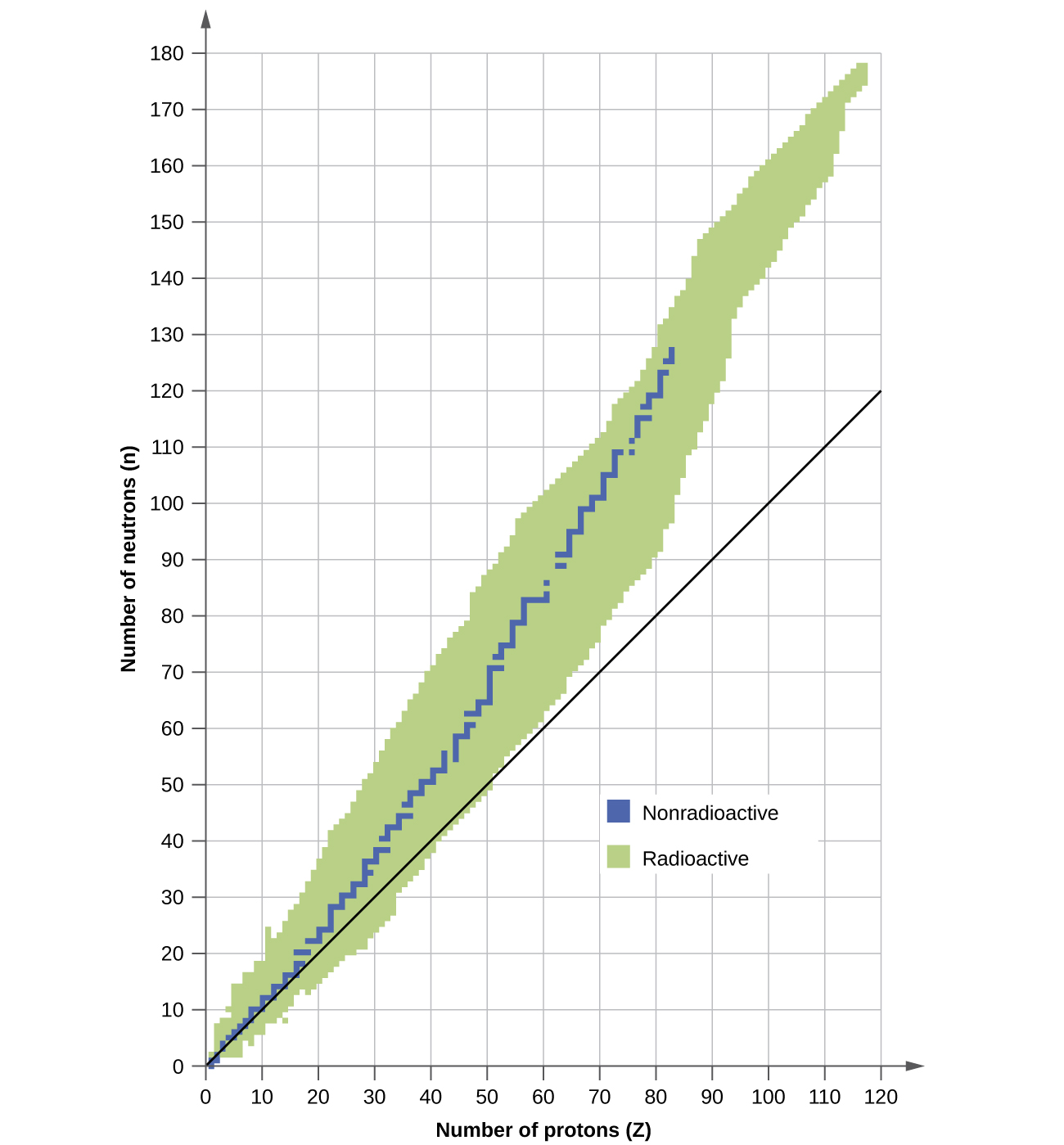
E - Stable nuclei generally have more neutrons than protons. Heavy nuclei with an even number of protons and an even number of neutrons are due to Pauli exclusion principle very stable thanks to the occurrence of paired spin. Nuclides containing even numbers of both protons and neutrons are most stable and this.
Which one of the following thicknesses of lead would be least effective in stopping β rays. On the other hand nuclei with an odd number of protons and neutrons are mostly unstable. CNucleus A is the most stable and nucleus D is not stable.
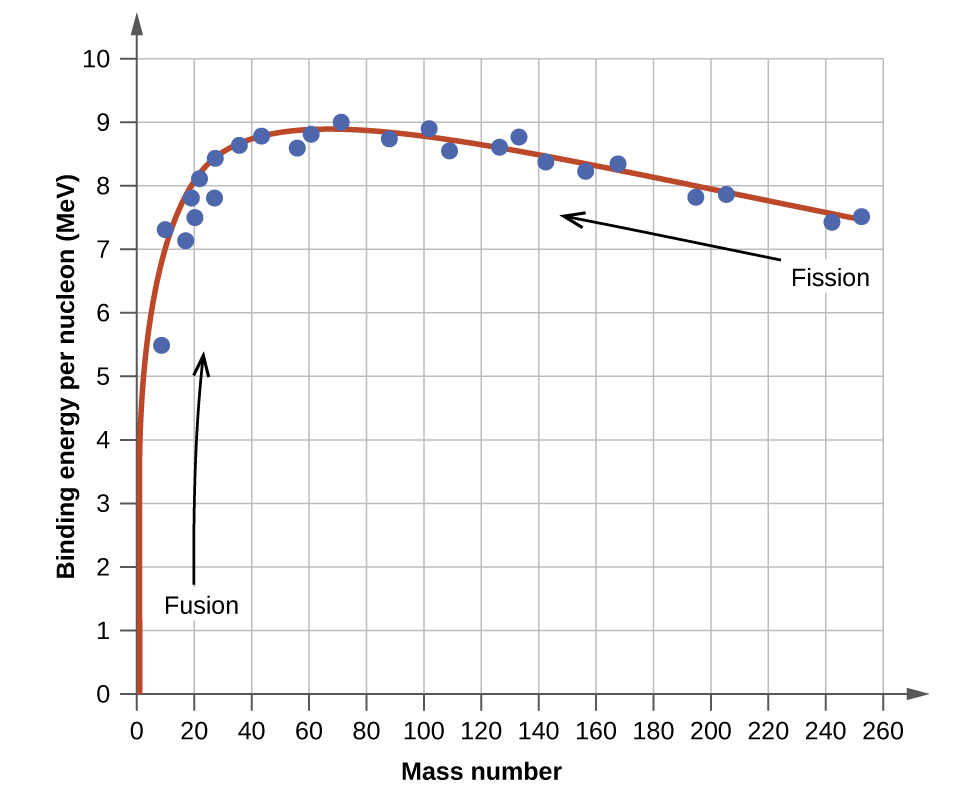
CAs the mass number A increases the binding energy per nucleon in a nucleus increases. Heavy hydrogen 2H1 sometimes called deuterium 2D1 has a. Some artificial radioactive isotopes can be prepared by bombarding stable nuclei with.
Nuclei with magic numbers usually tend to be more stable. All travel at the same speed. Nucleus 1 is stable.
Heavy nuclei are stable only when they have more neutrons than protons. Lighter nuclei have np ratio around 1. Which of the following processes produces nuclei of lower mass than the reactants.
Nucleus III is the most stable and nuclei IV and V are the least stable. The average binding energy for most of the nuclei is in the vicinity of 8 MeV. Representations of three nuclei with neutrons gray and protons purple are shown below.
See the related Wikipedia linkLarger atomic nuclei. Which statement is true regarding the stability of these nuclei. BEmission of α particle is normally followed by emission of γ rays.
Solutions for Chapter 24 Problem 148P. All the elements with an atomic number of more than 82 are unstable and radioactive irrespective of the number of neutrons. The three type of emission that originate from the nucleus are.
These types of nuclei are said to be radioactive and the emission is called radioactivity. A Phosphorus A31Z15 b Cobalt A59Z27 c Tungsten A184Z74 d Thorium A232Z90. If a nuclei has even numbers of nucleons its generally more stable.
ANuclear fission is normally followed by emission of β particles. BNucleus C is stable whereas nuclei A B and D are not. Nuclei with certain numbers of nucleons known as magic numbers are stable against nuclear decay.
Be 10 nuclei is unstable due to high np ratio which is 410. Thus heavy nuclei are neutron-rich compared to lighter nuclei. Which of the following lists ranks nuclear radiation from most massive to least massive.
A - 004 mm. C - gamma rays. This is the only nucleus without a neutron.
These numbers of protons or neutrons 2 8 20. As you go to heavier nuclei like uranium for instance thenucleus gets less stable. Iron has the maximum average binding energy 879 MeV and thus its nucleus is thermodynamically most stable.
Hydrogen 1H1has one proton and no neutron. B The light nuclei with A 20 are least stable. As said the stability of anucleus is influenced by the numbers of protons and neutrons in it.
Consider the following statements. On the other hand nuclei with an odd number of protons and neutrons are mostly unstable. In contrast the decay of the excited nuclear.
Nuclei I and II are not stable and nuclei IV and V are the most stable D. The unstable nuclei in order to become stable nuclei emit particles andor electromagnetic radiation. The ground state of this particular nucleus tantalum-180 is radioactive with a comparatively short half-life of 8 hours.
10 protons 12 neutrons because the closer the neutrons to the number of protons the more stable. Nuclei more massive than the Bi nucleus of bismuth A209Z83 are unstable and hence radioactive. DNuclei A and B are stable but nucleus B is more stable than nucleus A.
Correct option is B 4. Nuclei having binding energy per nucleon very near to 8 MeV are more or less stable. The first 80 elements of the periodic table have stable isotopes.
Which one of the following types of nuclear radiation is not affected by a magnetic field. Stable lightweight nuclei start the process.

Atom Rutherford S Nuclear Model Britannica
3 1 Nuclear Chemistry And Radioactive Decay Problems Chemistry Libretexts

Odd Odd Nuclei An Overview Sciencedirect Topics

Heavy Nuclei An Overview Sciencedirect Topics
21 1 Nuclear Structure And Stability Chemistry

Odd Odd Nuclei An Overview Sciencedirect Topics

Odd Odd Nuclei An Overview Sciencedirect Topics

0 Comments